Overview
- Summary
- Established in 1995, Mega Medical Co., Ltd. is a specialized medical device venture company that has successfully localized ENT treatment equipment, achieving the top market share in Korea. The company manufactures navigation surgical equipment, ENT treatment units, diagnostic imaging systems, and respiratory therapy equipment, expanding into the dermatology/obesity market since 2005 to provide total solutions.Established in 1995, Mega Medical Co., Ltd. is a specialized medical device venture company that has successfully localized ENT treatment equipment, achieving the top market share in Korea. The company manufactures navigation surgical equipment, ENT treatment units, diagnostic imaging systems, and respiratory therapy equipment, expanding into the dermatology/obesity market since 2005 to provide total solutions.
- Key Products/Technologies
- ENT Total Treatment unit (NET-600 Series, NET-3000 Series, NET-4000, NET-1100): Comprehensive treatment equipment for ENT, including various models such as NET-600A, NET-600B, NET-600C, NET-3000A, NET-3000C, and NET-3000D.ENT Total Treatment unit (NET-600 Series, NET-3000 Series, NET-4000, NET-1100): Comprehensive treatment equipment for ENT, including various models such as NET-600A, NET-600B, NET-600C, NET-3000A, NET-3000C, and NET-3000D.
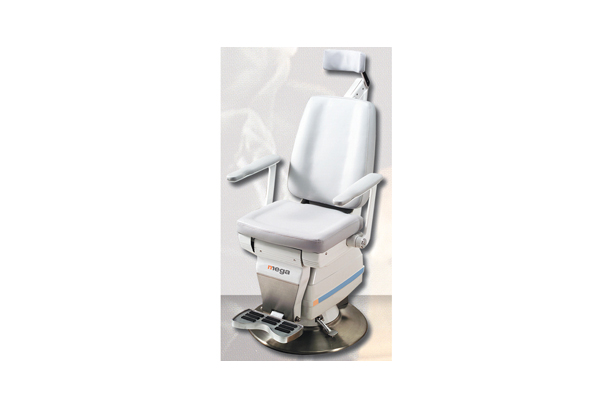
- ENT Chairs (NET-1500E, NET-A,B,C, NET-1500D): Specialized ENT chairs designed for patient comfort during examinations.ENT Chairs (NET-1500E, NET-A,B,C, NET-1500D): Specialized ENT chairs designed for patient comfort during examinations.
- Microscope (MHL-002, Micro-100): Microscopic equipment for precise diagnosis and surgery.Microscope (MHL-002, Micro-100): Microscopic equipment for precise diagnosis and surgery.
- ENT NAVIGATION System (Naviol): Advanced medical equipment providing 3D CT or MRI images for sinusitis surgery, guiding the precise location of surgical instruments, enhancing surgical accuracy and safety.ENT NAVIGATION System (Naviol): Advanced medical equipment providing 3D CT or MRI images for sinusitis surgery, guiding the precise location of surgical instruments, enhancing surgical accuracy and safety.
- STROBOSCOPY: Equipment for imaging the larynx and segmenting it into slow-motion cuts to aid in identifying affected areas.STROBOSCOPY: Equipment for imaging the larynx and segmenting it into slow-motion cuts to aid in identifying affected areas.
- FULL HD Visual System (FULL-HD, CQ-2000CV, DUAL CAMERA, NET-260SLCB FHD, VS-3CL FHD): High-performance imaging system developed for the localization of endoscopic equipment, supporting accurate surgery and diagnosis with clear image quality.FULL HD Visual System (FULL-HD, CQ-2000CV, DUAL CAMERA, NET-260SLCB FHD, VS-3CL FHD): High-performance imaging system developed for the localization of endoscopic equipment, supporting accurate surgery and diagnosis with clear image quality.
- Eustachian Tube Disease Treatment Equipment (Navilloon-e, Eustacure): Includes the Eustachian tube balloon catheter (Navilloon-e), with the company being the first in Korea to introduce Eustachian tube balloon dilation.Eustachian Tube Disease Treatment Equipment (Navilloon-e, Eustacure): Includes the Eustachian tube balloon catheter (Navilloon-e), with the company being the first in Korea to introduce Eustachian tube balloon dilation.
- Respiratory Therapy Equipment (RS-3000 RESPY): Equipment for treating respiratory diseases.Respiratory Therapy Equipment (RS-3000 RESPY): Equipment for treating respiratory diseases.
- Core Advantages
- Specialization in ENT treatment equipment with top domestic market share: Achieved the leading market position in Korea by successfully localizing ENT treatment devices since its establishment in 1995.Specialization in ENT treatment equipment with top domestic market share: Achieved the leading market position in Korea by successfully localizing ENT treatment devices since its establishment in 1995.
- Continuous R&D investment and operation of a corporate research institute: Invests over 10%, sometimes up to 25%, of annual revenue in R&D, developing cutting-edge products like ENT NAVIGATION, STROBOSCOPY, and FULL HD imaging systems through its affiliated research center.Continuous R&D investment and operation of a corporate research institute: Invests over 10%, sometimes up to 25%, of annual revenue in R&D, developing cutting-edge products like ENT NAVIGATION, STROBOSCOPY, and FULL HD imaging systems through its affiliated research center.
- Capability to provide total solutions and expand business areas: Beyond manufacturing ENT equipment, the company has expanded into the dermatology/obesity market, offering total solutions (Cell-Q COMBI I, Cell-Line Obesity Clinic) for obesity clinic operations.Capability to provide total solutions and expand business areas: Beyond manufacturing ENT equipment, the company has expanded into the dermatology/obesity market, offering total solutions (Cell-Q COMBI I, Cell-Line Obesity Clinic) for obesity clinic operations.
- Global market competitiveness and overseas branch operations: Exports to over 40 countries worldwide, operates overseas branches in China (Beijing), USA (NJ), and Europe (Greece), participates in 15 international exhibitions annually, and competes with global companies through competitive pricing and superior product quality.Global market competitiveness and overseas branch operations: Exports to over 40 countries worldwide, operates overseas branches in China (Beijing), USA (NJ), and Europe (Greece), participates in 15 international exhibitions annually, and competes with global companies through competitive pricing and superior product quality.
- Successful localization of advanced medical technology: Achieved successful domestic development of Full-HD imaging systems, offering reasonable prices and superior performance compared to imported equipment, and playing a pioneering role by introducing Eustachian tube balloon dilation in Korea.Successful localization of advanced medical technology: Achieved successful domestic development of Full-HD imaging systems, offering reasonable prices and superior performance compared to imported equipment, and playing a pioneering role by introducing Eustachian tube balloon dilation in Korea.
- Target Industrie
- Medical device manufacturingMedical device manufacturing
- ENT treatmentENT treatment
- Respiratory therapyRespiratory therapy
- Dermatology/Obesity treatmentDermatology/Obesity treatment
- Neurosurgery (using navigation systems)Neurosurgery (using navigation systems)
- Radiology (using imaging systems)Radiology (using imaging systems)
- Internal medicine (using imaging systems)Internal medicine (using imaging systems)
- Pediatrics (using imaging systems and respiratory equipment)Pediatrics (using imaging systems and respiratory equipment)
- Family medicine (using imaging systems)Family medicine (using imaging systems)
- Ophthalmology (using imaging systems)Ophthalmology (using imaging systems)
- Major Markets
- China, Middle EastChina, Middle East
- Turkey, Greece, Other European countriesTurkey, Greece, Other European countries
- USAUSA
- Certifications/Patents
- ISO 9001 certificationISO 9001 certification
- ISO 13485 certificationISO 13485 certification
- EN 46001 certificationEN 46001 certification
- KGMP (Korean Good Manufacturing Practice for Medical Devices) certificationKGMP (Korean Good Manufacturing Practice for Medical Devices) certification
- CE Mark certificationCE Mark certification
- Patent for wireless medical imaging diagnostic devicePatent for wireless medical imaging diagnostic device
- FDA approval (7 items including ENT UNIT, CHAIR, imaging devices)FDA approval (7 items including ENT UNIT, CHAIR, imaging devices)
- Awarded 'Export Tower of 1 Million Dollars' (2004)Awarded 'Export Tower of 1 Million Dollars' (2004)
- Awarded Minister of Commerce, Industry and Energy Award (2004)Awarded Minister of Commerce, Industry and Energy Award (2004)
- Awarded Public Procurement Service Administrator Award (2004)Awarded Public Procurement Service Administrator Award (2004)
- Awarded Incheon Mayor's Award (2004)Awarded Incheon Mayor's Award (2004)
- Selected as Technology Innovation Small and Medium Business (INNO-BIZ) (2004)Selected as Technology Innovation Small and Medium Business (INNO-BIZ) (2004)
- Selected as Inno-Biz New Technology Company (Technology Evaluation Center) (2005)Selected as Inno-Biz New Technology Company (Technology Evaluation Center) (2005)
- Registered 5 trademarks including medical inhalers (2005)Registered 5 trademarks including medical inhalers (2005)
- Designated as CLEAN workplace (Korea Occupational Safety and Health Agency, 2005)Designated as CLEAN workplace (Korea Occupational Safety and Health Agency, 2005)
- Selected as World-Class Product (2006)Selected as World-Class Product (2006)
- Acquired Korea Service Quality Excellence Company certification (Ministry of Knowledge Economy, 2009)Acquired Korea Service Quality Excellence Company certification (Ministry of Knowledge Economy, 2009)
- Selected as Promising Small and Medium Business in Gangwon (2010)Selected as Promising Small and Medium Business in Gangwon (2010)
- Selected as Good Company to Work in Our Region (2011)Selected as Good Company to Work in Our Region (2011)
- Awarded Minister of Health and Welfare Commendation (2017, CEO Kim Byung-jang)Awarded Minister of Health and Welfare Commendation (2017, CEO Kim Byung-jang)
- Own equipment 'Navilloon-e (Eustachian tube balloon catheter)' passed new medical technology assessment (2019)Own equipment 'Navilloon-e (Eustachian tube balloon catheter)' passed new medical technology assessment (2019)
Introduction
Key Products
Location
439 Bongeunsa-ro, Gangnam District, Seoul, South Korea
클릭하여 위치 살펴보기