This product is manufactured by
BIONET
Manufacturer information

BIONET
Medical equipment manufacturer, Bio-signal products, ECG(EKG), Patient monitoring, Fetal monitoring devices and more
Inquiry
How to order
Problem with product info?
Update request
Manufacturer
BIONET
Product Type
Machine
Brand
-
SKU
19329
Product Name
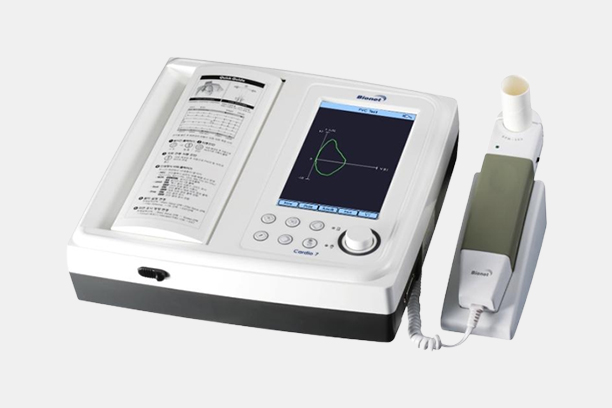
Electrocardiograph
Model Name
CardioXP
Size
-
Weight
-
Product Details
More products

Pulse OxymeterOxy9Wave

Patient MonitorBM1Vet

24시간 신호측정기CH-100

Infsion PumpPION Syringe

Infsion PumpPION TCI

Veterinary Mobile Ultrasound ScannerMU1V

Mobile Ultrasound ScannerMU1

Fetal central SystemFC Central II

Fetal MonitorFC700

Fetal MonitorFC1400

Patient Monitoring SystemBM Central

Patient MonitorBM1

Patient MonitorBM3

Patient MonitorBM5

Patient MonitorBM7

ElectrocardiographCardioCare2000
1/4